Friday, July 30, 2004
From Medscape: Caring for Patients in a Malpractice Crisis: Physician Satisfaction and Quality of Care:
Physicians across the country have politically mobilized in response to dramatic increases in medical malpractice insurance premiums, particularly for high-risk specialists. By the American Medical Association's (AMA s) reckoning, about two-thirds of U.S. states are now in the midst of a "malpractice crisis" or showing signs of trouble. Nowhere is the problem more acute than in Pennsylvania, where several insurers have exited the market and premiums for coverage through the remaining insurers have increased dramatically.The paper starts out with a very good question:
To investigate the effects of the malpractice crisis on patient care, we conducted a series of key-informant interviews with representatives from Pennsylvania physician groups, hospitals, and insurers, followed by a mail survey of 824 Pennsylvania physicians in high-risk specialties. This paper presents findings concerning the effects of the liability crisis on specialists' satisfaction and quality of care.
Physician satisfaction is often neglected or discounted as self-serving in policy debates. In this paper we outline a framework for understanding why physician satisfaction matters for patient care and what factors influence it. We then report on how the malpractice crisis appears to have affected satisfaction in Pennsylvania and explore the implications for quality of care. Our findings from Pennsylvania are not nationally generalizable, but they do provide a lens into the environment in states in severe malpractice crisis -- a point at which several states have already arrived, and toward which many others appear to be headed.
Why Does Physician (Dis)satisfaction Matter?Sounds like a vicious cycle to me. Happy physicians seem to engage in practices that lead to better outcomes. Better outcomes, one hopes, should keep physicians from being sued. Unhappy doctors that engage in "questionable prescribing practices" may be setting themselves up for an additional title, that of "defendant". The study looks at the effects of liability concerns on the following areas of practice:income, relationships, autonomy, practice environment, and market environment. The report contains quotes from survey participants, which do a good job of summing things up. Let us have a look:
Hard evidence is lacking for some of the irritants that cause grumbling among physicians, but professional dissatisfaction deserves policy attention if it has damaging consequences for patients. Empirical studies have identified associations between physician satisfaction and a variety of measures of quality of care. For example, patients of physicians with higher levels of job satisfaction have exhibited superior adherence to medical treatment. Satisfied physicians tend to be more attentive to patients and to have higher levels of satisfaction among their patients. Physician dissatisfaction, on the other hand, has been linked to riskier prescribing practices. Dissatisfied physicians are also more likely to leave clinical practice or relocate, disrupting continuity of care and jeopardizing access to services in underserved regions.
When financial stress is a source of dissatisfaction, physicians may change the insurance mix of their patients, increase patient volume, and reduce support services. Physicians dissatisfied with liability risks and costs may also take specific steps to reduce their exposure, such as restricting scope of practice, avoiding high-risk patients, and engaging in "defensive medicine."
Income:
I think if it were just the malpractice situation that added financial burden, it could be absorbed. We'd raise our rates to make up for that. But we have no way of passing on that extra cost to our consumers. And so, [reimbursement and insurance costs] both play a role.Nowadays the math doesn't work in one's favor:
I started alone in 1984, at which time my malpractice insurance was $18,000 and most people were getting $2,800 to deliver a baby. Today, the doctors are paying between $100,000 and $140,000 for malpractice [insurance], almost every patient is in an HMO, and we're getting $1,600 to deliver babies.
Relationships:
I heard a doctor say to a group of residents, "Every patient that comes in my office is a potential plaintiff, and that's the way I look at it." In my view, that's much more devastating to the health care system than [physicians relocating out of state].
When you are constantly looking over your shoulder and thinking that any less-than-perfect outcome is going to result in a lawsuit, it's not exactly the best psychological environment to try to concentrate on what you are doing with the patient.
Autonomy:
Physician overhead is going up, reimbursement is going down, and doctors need to figure out how to make up this difference by either working longer hours, buying fewer medical supplies, or cutting down on certain high-risk procedures to reduce malpractice rates.A very salient point. This could mean that the frequently-cited "exodus" of physicians form certain areas may be a late symptom rather than an early sign. This turn into another self-fulfilling prophecy. That is, volume may come at the expense of safety:
The more business [physicians] do, the more opportunity they have to pay their premiums. So access is going to be a very late victim. The first victim is going to be quality of care, in terms of how many patients you see an hour, the amount of time you give them.
"Doctors are delivering more babies per month than they should be," one head of a specialty society said. "They have to do it in order to generate enough money to maintain something of a lifestyle -- not their old lifestyle, but to just stay alive."So, in other words, they have to work more than eighty hours a week. There is more about the changes in market and practice environment. Mainly the respondents anticipate laying off staff. Many others have become hospital employees, joined group practices, or have restricted the amount of "high risk" medicine they practice. All factors that play into job satisfaction.
So again, why does physician dissatisfaction matter? Sounds like a great deal of whining to me. Rich, spoiled, crybaby physicians boo-hooing over their lack of "job satisfaction". Well it is important because any politician or governmental body that attempts to change (or not change) the health insurance, liability insurance, or access to care situation in this country ignores physician stakeholders at their own peril. Want examples? Start with the failed health care reform from the Clinton administration:
Moreover, the task force had neglected to identify the major stakeholders and seek “buy-in” for its health care plan. By excluding them—the insurance industry, the pharmaceutical industry, physicians’ groups, large and small employers, the media, and a multitude of others—from the planning process, the task force created a huge, powerful, and well-financed adversary.and finish with the recent goings-on in West Virginia:
In the already overburdened Level I trauma center in the state capital of Charleston, the orthopaedic surgeons finally rebelled, faced with an avalanche of patients being transferred to their institution around the clock from outlying communities that found themselves without orthopaedic coverage or the courage to assume the risk of liability. The surgeons announced the decision to stop taking trauma call. Immediately thereafter, the hospital was downgraded to a Level III trauma center. The public perception based on the media coverage was that the hospital was essentially closed for all critical patients. The state capital was in an uproar as the legislature was about to convene for the 2003 session.....a large group of northern panhandle surgical specialists stopped all their elective cases. Hospitals in the area, which were already struggling under inadequate reimbursement, began to suffer mounting losses in income. The hospital association’s involvement in the lobbying effort intensified dramatically.....Critical portions of the tort reform bill include a $250,000 cap on noneconomic damages and a $500,000 cap on all damages for treatment of emergency conditions for patients who receive care at a designated trauma center. Joint liability has been eliminated, and each individual defendant bears liability equal to his or her percentage of fault. Collateral payments, which had not been allowed in court before, may now be presented. The “ostensible agency theory” of liability was abolished. Additionally, a committee was established to develop a patient injury compensation fund to provide for economic damages that exceed financial limits set in the bill.
I am sure it improved their job satisfaction.
|
Thursday, July 29, 2004
From The New York Times:Study Shows M.R.I. Scans Are Better Finding Tumors:
In women at high risk of breast cancer, new research suggests that M.R.I. scans find nearly twice as many tumors as mammograms do, but that they cost a lot and trigger more unneeded biopsiesSo goes the lead paragraph about a study that was published in this week's New England Journal of Medicine:Efficacy of MRI and Mammography for Breast-Cancer Screening in Women with a Familial or Genetic Predisposition
Background:The value of regular surveillance for breast cancer in women with a genetic or familial predisposition to breast cancer is currently unproven. We compared the efficacy of magnetic resonance imaging (MRI) with that of mammography for screening in this group of high-risk women.The women in this study all had ,at minimum, a fifteen percent lifetime risk of breast cancer. The patients were stratified in groups according to risk, ranging from mutation carriers (BRCA) to medium (>15%) risk. As seen above MRI was twice as likely to detect a tumor as mammography and those found were smaller with fewer lymph node mets. The tumors in the mutation group were larger and more were ER/PR negative than in the other groups. In one area, the diagnosis of DCIS, mammography was superior:
Methods: Women who had a cumulative lifetime risk of breast cancer of 15 percent or more were screened every six months with a clinical breast examination and once a year by mammography and MRI, with independent readings. The characteristics of the cancers that were detected were compared with the characteristics of those in two different age-matched control groups.
Results: We screened 1909 eligible women, including 358 carriers of germ-line mutations. Within a median follow-up period of 2.9 years, 51 tumors (44 invasive cancers, 6 ductal carcinomas in situ, and 1 lymphoma) and 1 lobular carcinoma in situ were detected. The sensitivity of clinical breast examination, mammography, and MRI for detecting invasive breast cancer was 17.9 percent, 33.3 percent, and 79.5 percent, respectively, and the specificity was 98.1 percent, 95.0 percent, and 89.8 percent, respectively. The overall discriminating capacity of MRI was significantly better than that of mammography (P<0.05). The proportion of invasive tumors that were 10 mm or less in diameter was significantly greater in our surveillance group (43.2 percent) than in either control group (14.0 percent [P<0.001] and 12.5 percent [P=0.04], respectively). The combined incidence of positive axillary nodes and micrometastases in invasive cancers in our study was 21.4 percent, as compared with 52.4 percent (P<0.001) and 56.4 percent (P=0.001) in the two control groups.
Conclusions: MRI appears to be more sensitive than mammography in detecting tumors in women with an inherited susceptibility to breast cancer.
Another important matter that we addressed was the best method for detecting carcinoma in situ. Our study showed that mammography had a higher sensitivity than MRI for detecting ductal carcinoma in situ: 83 percent (five out of six cancers detected), as compared with 17 percent (one out of six) for MRI (P=0.22).This is likely to have a lot of women asking for MRIs.
Some issues I have with the study:
With a follow-up of only 2.9 years, the question remains open whether the early detection that is available with MRI leads to increased survival. In fact the "early detection = increased survival" debate still goes on about mammography.
Not every community MRI scanner can perform breast MRI. Specialized coils are needed. The few I have ordered in the past have had to be sent to an academic center.
There is a sacrifice of specificity:
A drawback of MRI screening is that it has a lower specificity than mammography, and as a result, MRI will generate more findings judged as uncertain, which require short-term follow-up or additional investigations In our study, screening by MRI led to twice as many unneeded additional examinations as did mammography (420 vs. 207) and three times as many unneeded biopsies (24 vs. 7).All which leads to increased cost and anxiety on the part of the patient. Especially in a group that is unlikely to engage in a pattern of "watchful waiting'.
And finally, the cost of MRI:
An M.R.I. scan costs $700 to $1,000 - about 10 times the cost of a mammogramWho will pay?
Overall a fairly good study. Hopefully this population will be followed long-term to evaluate for survival benefit.
|
Wednesday, July 28, 2004
From The New Yorker:
ALL THAT YOU CAN BE
There has been a great deal of speculation recently that the government might reinstate the draft at some point, in order to replenish the nations armed forces. Military and government officials have, for the most part, dismissed such talk. As Secretary of Defense Donald Rumsfeld said in an interview the other day, Were perfectly capable of increasing the incentives and the inducements to attract people into the armed services. For years, the military has offered its recruits free tuition, specialized training, and a host of other benefits to compensate for the tremendous sacrifices they are called upon to make. Lately, many of them have been taking advantage of another perk: free cosmetic surgery.....This got exposure from The Agitator, the Pittsburgh Tribune-Review (of Teresa Heinz-Kerry "shove it" fame) and from The Corner at NRO. Shocking, isn't it. Until you look at the numbers in context, as this reader of The Corner did:
A Defense Department spokeswoman confirmed the existence of the plastic-surgery benefit. According to the Army, between 2000 and 2003 its doctors performed four hundred and ninety-six breast enlargements and a thousand three hundred and sixty-one liposuction surgeries on soldiers and their dependents. In the first three months of 2004, it performed sixty breast enhancements and two hundred and thirty-one liposuctions.
However, there are some points of contention regarding the fact impaired New Yorker-via-Reuters story you referenced. I don't have access to the New Yorker article, but have seen it summarized on line. From these, I would like add some facts that make the story pretty dang benign and hardly the shocking waste of taxpayer dollars the spin of the article seems to be. The tenor that Sergeant Baggadonuts and/or Mrs. Major Zotz can just waltz over to the nearest military hospital and get nipped, tucked, vacuumed, or pumped up, is both bogus and irksome. I am a little weary of lies and misdirection regarding the military.I agree that this is a tempest in a teapot. Given the numbers above, the procedures don't seem to be all that out of line. There are probably even more dependents who have had cosmetic procedures done by civilian plastic surgeons. However I take issue with reasons #1-4 above, as a search of the ACGME site revealed no accredited plastic surgery programs that were affiliated with a military hospital, although there have been some in the past. A small point, I know but the official line of the Army sounds much like it:
1. The Army, Navy, and Air Force have teaching hospitals.
2. These hospitals have plastic surgery programs.
3. For these programs to be accredited, the residents must perform the same procedures their civilian counterparts perform.
4. To become board certified and maintain currency, military plastic surgeons must perform the same procedures as their civilian counterparts.
4. The article mentions that between 2000 and 2003, there were 496 breast enlargements and 1361 lipos done on "soldiers" (only Army personnel are Soldiers) and their dependents. Assuming they were done at a constant rate, that is a whopping 165 breast enlargements and 457 lipos per year.
5. There are about 1,400,000 active duty military, and about an average of 3 dependents/servicemember. Completely disregarding eligible reserve component personnel and their dependents, as well as eligible retirees, that makes an eligible population of over 4, 200,000.
Do the math, and as you can see, this falls damn short of a cosmetic surgery stampede. Show me any U.S. city of 4.2 million in which the plastic surgeons only do 165 breast enlargements and 457 lipos a year, and I'll show you a town with starving plastic surgeons, or with only one moderately busy. Show me a town of 4.2 million with only one plastic surgeon, and I'll show you a town outside of the U.S. I won't bore you with the hoops and wickets one has to go through to get someone one a rare referral for these or other "elective cosmetic" procedures - trust me, it isn't the same pulling out the Yellow Pages, turning to the physicians pages, picking an ad, and calling up old Dr. Young for an appointment - there has to be some kind of justification other then "I want it".
In addition, as odd as it may seem, military and their dependents do get sick and injured - if a spouse or a soldier needed, for example, a breast reconstruction after mastectomy, I think most sentient persons (I know that whittles the pool down a fair bit) would agree that docs who have actually performed a few cases, even if those were strictly cosmetic, would be preferred over those doing OJT. Same thing goes for nose jobs or any other "elective cosmetic" procedure - the bottom line is that skills maintained by doing these procedures are helping heal servicemembers injured in combat, past and present.
The Armys rationale is that, as a spokeswoman said, the surgeons have to have someone to practice on.It's not just a job, it's an adventure.
|

From the Fark Photoshop contest "celebs who are dead-ass wrong for a superhero role".
Does kind of fit Senator Edwards, though...
|
Tuesday, July 27, 2004
Dr. Smith is holding court at Overlawyered this week. Today she shares the story of a physician named in a suit over a situation that occurred over a year after they left that hospital. The physician in question was eventually dropped. After lamenting the lack of consequences for the attorney in question the author makes the point that a case doesn't have to be lost for liability premiums to rise:
I'd like to reiterate, had I not done things the way I had, but rather called my insurer and had them handle it, it would have probably cost tens of thousands of their dollars to figure this out. Further, I would have had an open claim on my record and my rates would have been jacked up for several years...all because a lawyer wasn't held accountable up front for reckless behavior.Even when a suit hasn't been filed yet, costs may accrue. I have gotten demand letters from lawyers representing patients I had peripheral involvement with (you put in a central line in this patient that had other problems, give us a million dollars) that I have spoken to my liability carrier about. This is an expenditure of time on my part and money (to open a file, call around, ect) on theirs.
Another trick I have noticed some lawyers engaging in is filing suit against the main characters and also various "John Does". I guess those are reserved seats for those poor souls who are found out during discovery. So, for example, I am deposed in a case which currently I am not named in, and the statute of limitations has expired. But several "John Does" are named. What to do? Have my liability carrier retain counsel for me, of course. Heaven forbid I become "John Doe #1" |
Monday, July 26, 2004
Senator John Kerry:

and....
Woody Allen:

I report, you decide.
Top image via the Professor
|
This editorial appeared in last week's New England Journal of Medicine
The Price Tag on Progress Chemotherapy for Colorectal Cancer. The moderator of the weekly cancer conference at Big Hospital gave a little talk about it today. It seems that we are paying a lot for a little time:
Better systemic therapy has considerably improved prognosis. Without chemotherapy, the median duration of survival among patients with metastatic colorectal cancer was eight months. With fluorouracil, it increased to 12 months. After 2002, the availability of three cytotoxic agents (fluorouracil, irinotecan, and oxaliplatin) further extended the median survival to 21 months. Although clinical-trial data are not yet mature, bevacizumab and cetuximab are likely to prolong median survival beyond 21 months. But despite the panoply of options, treatment remains palliative, and there is not yet evidence that the new therapies increase cure rates.So for a regimen that is not even curative, the costs of the drugs rises rapidly. The next paragraph brings it into more perspective:
In the wake of the optimism generated by recent trial results, patients experience sticker shock when they encounter the prices of chemotherapy drugs. Physicians find themselves in the undesirable position of having to help patients make decisions about whether the potential clinical benefits warrant the financial strain that even the copayments for these medications may create. Consider the drug costs for a patient with colorectal cancer who is 170 cm tall, weighs 70 kg, and receives chemotherapy for eight weeks the duration typically required to determine response. The Mayo Clinic regimen (fluorouracil and leucovorin) costs $63. The FOLFOX regimen (fluorouracil, leucovorin, and oxaliplatin) costs $11,889, and FOLFOX combined with bevacizumab costs $21,033 (see Table). The near-doubling of the median survival achieved over the past decade has been accompanied by a staggering 340-fold increase in drug costs just for the initial eight weeks of treatment.
The combination of irinotecan and cetuximab for second- and third-line treatment of metastatic colorectal cancer, as described by Cunningham et al. in this issue of the Journal (pages 337345), increases the median survival by 1.7 months. In the United States, the regimen costs approximately $30,790 for an eight-week course. Assuming that an average patient continues to receive treatment until the median time to progression, 8 months of front-line therapy followed by 4.1 months of irinotecancetuximab therapy would cost $161,000. In 2004, 32,000 people in the United States will receive a diagnosis of stage IV colorectal cancer, and recurrent metastatic disease will develop in an additional 24,000. The drug costs for an eight-week course of initial treatment for these 56,000 patients will be approximately $666 million or $1.2 billion with the addition of monoclonal-antibody therapy. These cost estimates are exclusively for drugs; they do not include the costs of preparation, administration and supervision, or supportive medications. They presume that every single milligram of drug is effectively utilized. Yet cetuximab, for example, is manufactured only in a 100-mg vial, and because the medication cannot be stored for long periods, leftovers go to waste, particularly in small practice environments.Other items not included are hospitalizations due to chemotherapy complications or other medications, such as anti-emetics, that are used as well. How many screening colonoscopies could that kind of money pay for? Not only can the 1.7 months of increased survival be expensive, but what sort of quality can one have? Some may be well enough to take a trip or see a family member's graduation or wedding, all priceless events, but many others will spend that time nauseated, weak, and anemic. To throw another wrench in the works, monoclonal antibodies are now being used in the treatment of advanced lung cancer, a disease that affects close to three times the number of patients that advanced colorectal cancer does.
Then my evil, cynical side came out. Dr. Smith has already sung the praises of the NEJM for raising this issue and calls for future examinations of cost/benefit ratios. What would have happened if an editorial asking the same questions about a politically-sensitive disease such as breast cancer or HIV/AIDS had been published? Would it have been published? The hue and cry would have been impressive to say the least. Lastly:
As a society, we are reluctant to systematically deny access to expensive treatments that extend life by only a few weeks, but the morality of refusing to make deliberated choices is itself questionable.|
Sunday, July 25, 2004
DB linked to this New York Times article today:
In a Shift, Bush Moves to Block Medical Suits
The Bush administration has been going to court to block lawsuits by consumers who say they have been injured by prescription drugs and medical devices.
The administration contends that consumers cannot recover damages for such injuries if the products have been approved by the Food and Drug Administration. In court papers, the Justice Department acknowledges that this position reflects a "change in governmental policy," and it has persuaded some judges to accept its arguments, most recently scoring a victory in the federal appeals court in Philadelphia.
Allowing consumers to sue manufacturers would "undermine public health" and interfere with federal regulation of drugs and devices, by encouraging "lay judges and juries to second-guess" experts at the F.D.A., the government said in siding with the maker of a heart pump sued by the widow of a Pennsylvania man. Moreover, it said, if such lawsuits succeed, some good products may be removed from the market, depriving patients of beneficial treatments.
What if the company lies?
Ancure® system (Guidant)Should that be handled by the FDA, a lawsuit, or a criminal proceeding?
This device has a flexible, unsupported fabric graft prosthesis that is actively fixed in place on the ends by wire hooks that penetrate the vascular tissue. On 3/16/01, Guidant suspended production and announced a recall of all existing inventory. The company reported to the FDA that they had failed to report many device malfunctions and adverse events, including severe vessel damage associated with problems with the deployment of the device. There were also manufacturing changes that were not properly reported to the FDA. The manufacturer told FDA that an internal audit revealed problems with their complaint handling system, manufacturing quality systems, documentation procedures and training. The FDA is reviewing the firm's Corrective Action Plan that addresses these problems. Once we receive evidence that the firm has appropriately changed their systems and procedures, and the FDA has reviewed their regulatory submissions, we will assess whether the product can be returned to the market.
As I have pointed out previously the FDA is not immune to pressure placed on it by device manufacturers.
Employing the intellectually suspect "slippery slope" argument, shouldn't every item manufactured according to governmental regulations: airplanes, cars, boats, and fire-resistant children's sleepwear be immune from lawsuits?
One of DB's commenters suggests that such a system would eliminate or minimize the use of medications or devices in an "off label" fashion. In theory, a drug maker should not be held liable for "off label" uses since the company has not advocated those uses. The FDA does not look kindly on companies promoting "off label" uses for their medications, a lesson that cost Warner-Lambert $430 million to learn.
I believe that if the FDA approves a device or medication, based on honest information provided by the manufacturer and if the manufacturer provides honest and prompt reporting of adverse effects, then FDA approval should provide immunity from suits. The companies may have to give up some "proprietary" information. But if it can escape liability, they may be willing to do so.
|
Friday, July 23, 2004
Kevin provides a link and commentary to this article concerning the difference between weekday and weekend utilization of certain diagnostic and therapeutic tests. The author of the study found that fiberoptic bronchoscopy, MRI, upper endoscopy, echocardiography, V/Q scanning, and cardiac catheterization were more likely to be performed on weekdays than on weekends. The study's author (as well as Kevin) opine that LOS could be shortened and outcomes improved if weekends could be treated like weekdays.
With the caveat that I don't have access to the full article, here are my thoughts:
There is a difference between "urgent" and "emergent" procedures. The definition of urgent in this paper by my reading seems to be "procedures performed on hospital inpatients". Of those listed I would consider V/Q scanning to be more urgent than the others listed. The others can fall into either camp.
The "well that's a Canadian hospital" argument doesn't apply since many of those services are rarely available in American hospitals.
As one who performs procedures from time to time I have to deal with the short staffing of hospitals on the weekends. Not only of nurses and techs but the unavailability of other physicians. This affects things in two ways:
There is less backup in case something goes wrong during or after a procedure. A patient that develops airway difficulty after an upper endoscopy done during business hours has many people with the skills and equipment to handle the problem available to tend to them. Over the weekend there may only be one person available to assist, and that person may not even be in house.
Physician availability is less. During the week if I am on call and doing an elective or urgent procedure and something comes in the ED, I can ask one of my partners to lend a hand. During the weekend they may not be available. That places limits on what can, or should be done. Imagine the situation of a surgeon performing a lap chole over the weekend and a ruptured AAA presents to the ED. The AAA patient expires, if the surgeon is sued what sort of defense does he have for not attending to an emergent patient while he performs what is essentially an elective procedure?
But what if the hospital was fully staffed? Given the current nursing shortage and the premium that would have to be paid to staff to work the weekend, to give up time with their friends and family, would likely surpass any LOS savings that could be achieved. The famous Baylor Plan pays nurses the equivalent of 40 hours for 24 hours of weekend work. And what of the physicians? Given the current enthusiasm for resident work restrictions and the growing emphasis on family and lifestyle issues among physicians of all levels, the likelihood of "every day a weekday" is remote at best. |
One of the most stressful times around a hospital is when the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) makes their triennial visit. Medical staffs have many more meetings, administrators stay busy teaching the staff to recite the "mission statement" on demand and the maintenance crews stay busy painting everything. It is felt by many to be annoying and intrusive. The surveys are tolerated mainly for two reasons: 1. Medicare funding is dependent on passing a survey and 2. It promotes patient safety. While the former is most assuredly true, the same cannot be said about the latter. From MSNBC: Hospital inspectors miss 'deficiencies'.
The private organization that clears hospitals to receive Medicare payments missed most problems later identified by state inspectors, potentially compromising patient safety, congressional investigators said Tuesday.It seems that while JCAHO can determines if a hospital participates in Medicare or not, the Center for Medicare Services (CMS) is forbidden by statute to set the conditions for JCAHO approval. CMS contracts with various state agencies to provide validation of JCAHO's methodology. From the GAO report (PDF):
The Joint Commission on Accreditation of Healthcare Organizations, made up mainly of health professionals, failed to find 167 of 241 serious deficiencies in a survey of 500 hospitals that were reviewed between 2000 and 2002, the Government Accountability Office said. The agency, Congress investigative arm, was formerly called the General Accounting Office.
JCAHOs pre-2004 hospital accreditation process often did not identify either hospitals with serious deficiencies or the individual serious deficiencies found by state survey agencies through CMSs validation program. In a sample of 500 JCAHO-accredited hospitals, state agency validation surveys conducted in fiscal years 2000 through 2002 identified 31 percent (157 hospitals) with serious deficiencies; of these, JCAHO did not identify 78 percent (123 hospitals) as having serious deficiencies. For the same validation survey sample, the majority of the serious deficiencies state survey agencies identified but JCAHO did not were in the physical environment COP category, which covers fire safety and prevention.In referring to the tables the percentage of "serious" deficiencies missed by JCAHO that were found by the state inspectors are falling, from a high of 85% in 2000 to 61% in 2002. Most of the issues were physical plant related:
Of the 167 serious deficiencies identified by CMSs validation program from fiscal year 2000 through 2002 but not detected by JCAHO, 87 were related to a hospitals physical environment, which includes life safety code standards on fire prevention and safety.Not surprising since I have yet to meet a fire Marshall or a building inspector on a JCAHO survey team. But JCAHO gets low marks on patient-care related items as well. The first column is the number of events found by state inspectors, the second column is those that JCAHO missed:
So deficiencies were found in things that are the big targets of a JCAHO survey : QA, nursing services and medical records. They did do well on "medical staff" issues, which from personal experience has a heavy emphasis on the credentials process.Physical environment 107 87
Quality of care
Anesthesia services 3 2
Discharge planning 2 2
Emergency services 2 2
Food and dietetic services 5 4
Governing body 16 7
Infection control 15 9
Laboratory services 1 1
Medical record services 7 4
Medical staff 10 1
Nursing services 17 10
Organ, tissue, and eye procurement 5 5
Outpatient services 1 1
Patients rights 10 9
Pharmaceutical services 14 9
Quality assurance 18 8
Radiologic services 1 0
Rehabilitation services 1 1
Respiratory care services 1 1
Surgical services 5 4
Total quality-of-care COPs 134 80
The folks at JCAHO do not take kindly to the findings:
Commission president Dennis OLeary said his group made sweeping changes to the accreditation process earlier this year. In our view, it is irresponsible to alarm the public using statistics that have little meaning, OLeary said in response to the GAO report.This is a reference to the "new" survey procedures. The GAO is reserving judgment:
The potential of JCAHOs new hospital accreditation process to improve the identification of serious deficiencies is unknown because it is too soon after its January 2004 implementation for a meaningful evaluation; in addition, JCAHOs testing of the new process was limited. CMS has not had the opportunity to complete its validation program for 2004 to determine whether JCAHO surveyors using the new process are missing serious deficiencies later identified by state agency validation surveys. While unannounced surveys, which are planned for implementation in 2006, have the potential to improve the detection of serious deficiencies, other features of the new process that JCAHO did not test before implementation may have imitations that could affect the potential of the new process to identify problems with patient care. JCAHOs pilot test of the new process had limitations, including using a ample of hospitals that volunteered for the pilot instead of using a random sample and self evaluating the results instead of using an independent entity.
So what does this mean?
Life will become more difficult for JCAHO. There will be pressure on congress to pass a law to give CMS a greater role in determining JCAHO's priorities and methodology. JCAHO may be able to put it off for a few years given the fact they have changed the way they conduct a surveys and the initiation of unannounced visits in the next few years. I am sure that those results will be scrutinized.
Either to comply with, or to avoid increased government involvement the JCAHO surveys will become more intrusive. They will start crawling through basements, counting lightbulbs at emergency exits while maintaining their constant vigilance of documentation of pain as the fifth vital sign and the elimination of QHS and QD as approved abbreviations.
And since we all know what rolls downhill, this will put additional pressure on medical and nursing staffs of hospitals. With the advent of unannounced visits the hospital will essentially be at an "at war" footing at all times.
|
Wednesday, July 21, 2004
Lifting a link from Mr. McBride I bring to you Radley Balko's A Few Questions for the Vice Presidential Candidates. Mr Balko asks both Senator Edwards and Vice-President Cheney some pointed questions:
Mr. Balko also asks Senator Edwards questions about late-term abortions and the race of judicial nominees. Important issues but not the purpose of this post. To further drive home the point of the first two questions I would like to add another:For Sen. John Edwards, Democratic nominee for vice president:
—As is well-known by now, you made your fortune representing plaintiffs in tort cases, mostly in medical malpractice cases. One of the consequences of huge rewards like those you have won has been skyrocketing malpractice insurance premiums for doctors and the exodus of doctors from certain medical specialties, such as OB-GYNs, heart surgeons and emergency room attendants. The resultant crisis in health care availability has hit particularly hard in the South, as medical insurers and doctors have abandoned much of the region. As someone who has presented himself as an advocate for the little guy, how do you reconcile the verdicts you've won with the effects those verdicts had on health care in the South?
—You specialized in seeking judgments for parents in cases of botched childbirth deliveries, which you argued resulted in cases of cerebral palsy. Two new studies released in 2003 show strong evidence that cerebral palsy is not likely caused by delivery room errors, but by genetics or prenatal infections. If medical science continues to exonerate delivery doctors from cerebral palsy cases, do you think the verdicts against the doctors you successfully sued should be reversed, or that your clients should return the money?
—You said in a campaign speech that we have “One America that pays the taxes, another America that gets the tax breaks,” and that in your administration, we’d have “no more tax breaks for CEOs who give themselves millions in top-hat pensions while giving no pensions at all to ordinary workers.” Yet, two years before you were elected to the Senate, you established a tax shelter to avoid paying $290,000 in Medicare taxes. Medicare is federally-funded healthcare for the poor. How do you explain your shirking of this duty to the poor you claim other wealthy people should observe?
You frequently portray your litigation career as standing up for the "little guy" and defend your large awards as a way for your clients to be provided for. Why did you then oppose the establishment of a "no-fault" program for cerebral palsy patients in North Carolina similar to the one in Florida?Certainly it was Senator Edwards' duty to provide the best case he could for his clients within the limits of the law. But if he was truly the populist he claims he is, why did he not take every case he was presented with? Why did he deny the Medicare program that $290,000 ? Where is the outrage? |
Tuesday, July 20, 2004
Over the weekend Mrs. Parker had us cleaning out the closet in the home office. In doing so we came across the operative reports I had dictated as a resident. They consisted of a 3 foot stack of 81/2 by 11 inch sheets. I had resisted the call to discard them in the past giving excuses that I may need the reports for privileges in the future. Given the amount of time I have been in private practice that argument rang hollow. So I spent several hours shredding that pile of paper (have to be HIPAA compliant, you know) .
What a trip through memory lane.
I would read a name and reflect back on that patient. The ones who we snatched from the edge of death, and the ones we accidentally pushed over the side. The old man with pancreatitis that we resuscitated for two straight days and thought we had turned the corner. Only to have him get caught up in a cycle of sepsis and hepatic encephalopathy and watch him die slowly over 3 months. The 25 year old with a severe liver laceration damage control laparotomy the first day and multiple trips to the OR. She lived to become addicted to narcotics.
I read of operations done with attendings and residents I would gladly let operate on my spouse or children, and those I wouldn't trust with my worst enemy.
I read of new and novel therapies that held promise in a journal article, but fell apart in the harsh light of reality.
I had touched these patients in ways their own loved ones had not, and I hope they were helped by it. Each of them taught me something over those five years and I was the better for it.
Five years of one's professional life in a 36X81/2X11 inch stack of paper. Just about 5 32 gallon lawn-and-leaf bags after it had gone through the shredder.
The trash guys didn't pick it up .
If you live in Georgia, don't forget to vote today.
|
Friday, July 16, 2004
Being on call five out of the last seven days has really socked it to me. Last night was the worst as I had four traumas come in at the same time:
23 y/o helmeted motorcylist with a dislocated left shoulder and abrasions to the palms of his hands.
30-ish non-english speaking man fell 35 feet from a scaffolding. He had a cerebral contusion and cervical strain.
25 y/o fell off the back of a truck. He had AMS at the scene and by the time he arrived EMS had the ambu-bag on him. No ED doc available so I got to intubate him. He had a depressed skull fracture and a cerebral contusion. Severely displaced clavicle fracture. The neurosurgeons took him to the OR.
His most impressive CT scan slice and his chest x-ray are provided for your viewing pleasure.
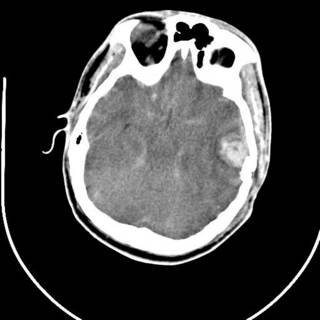
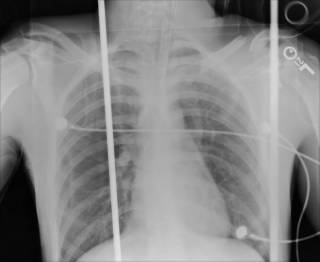
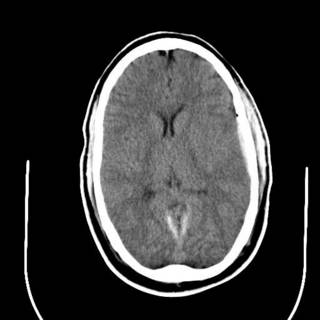
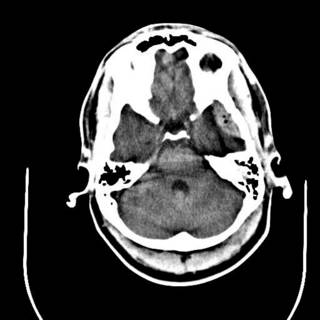
The last was a 21 year-old ATV versus tree. The tree won. He is now the proud owner of an ICP monitor. His CT scans:
While I agree wholeheartedly with Kevin about the changes at Medlogs, I am reserving my judgment on the new Blogger editor for the time being.
Well I'm going to grab a piece of the couch for a little nap. Have a nice weekend.
|
Wednesday, July 14, 2004
As Dr. Shazam keeps us up to date with her air ambulance adventures she and the crew have all done well. That is not always the case. From the Orangeburg, SC Times and Democrat: Four die when medical helicopter with accident victim crashes
The crash was so muffled early Tuesday morning, most people at the rest area along Interstate 26 thought it was just another wreck on the highway.The number of helicopter crashes are on the rise according to The Journal of Trauma Medical Helicopter Accidents in the United States: A 10-Year Review. (abstract free)
But trucker Johnny Williamson knew it was the crash of the medical helicopter he had watched take off less than a minute ago, carrying a woman with a broken leg to a Spartanburg hospital.
The woman, along with three medical workers from the Spartanburg Regional Healthcare System, were killed when the helicopter went down.....The medical helicopter crashed about 5:40 a.m. on a hiking trail behind a rest area along I-26, Newberry County Sheriff Lee Foster said. It was in the air less than a minute, the sheriff said.....The incident began around 4:30 a.m. when a state trooper found a woman in the median of I-26 who said she had been hit by an 18-wheeler. Her leg was broken and she had some bruises and scrapes to her chest. Paramedics checked her out, decided she needed to go to a trauma center and called for a helicopter, Foster said.
That isn't unusual in Newberry County, Foster said. Routinely, helicopters are called in when an ambulance might get stuck in rush hour traffic. Also, emergency workers can get patients to the hospital faster by flying and that's critical in trauma cases, the sheriff said.
From the discussion:
In all medical endeavors, safety of the patient and medical personnel has been a paramount concern. We initially reported a worrisome trend in U.S. medical helicopter accidents for a 5-year period. 5 Our study shows a marked increase in the number of medical helicopter accidents for the 10-year study period. In particular, over half (52%) of all accidents in the study period occurred during the last 3 years of the study (2000-2002). These results appear reflective of a trend first identified by the NTSB in 1988. The NTSB recognized an alarming increase in medical helicopter accidents in the 1980s. Specifically, there were 14 major medical helicopter accidents in 1986 that destroyed or significantly damaged 9% of the U.S. medical helicopter fleet. After this, they undertook a safety study of helicopter air operations and found an accident rate almost twice that of nonscheduled air taxi helicopters and a fatal accident rate 3.5 times greater. 6 After publication of their study, an improvement in medical helicopter accident rates occurred.See the graph:

Posted by Hello
Some research is asking the question: Is this trip really necessary?
The increased number of medical helicopter accidents we have reported is noteworthy in that several recent studies have shown that medical helicopters in the United States and other countries are overused. Shatney and colleagues reported a retrospective review of 947 consecutive trauma patients transported to their trauma center in the Silicon Valley of California, where they found that only 22.8% of study patients possibly benefited from helicopter transport. They further found that 33.5% of patients transported by helicopter were discharged from the emergency/casualty department and not admitted to the hospital. Eckstein and colleagues retrospectively evaluated helicopter transport of 189 pediatric trauma patients in the Los Angeles, California, area. They found that 85% of patients were considered to have minor injuries. Of the patients transported by helicopter in their study, 33% were discharged from the emergency/casualty department and not admitted to the hospital.The abstracts are here and here.
Despite the questions I believe that the use of helicopters for emergency medical transport of all kinds will increase over time. As smaller hospitals close entirely or close their ED's patients will have to be transported a greater distance to receive medical care. The increasing regionalization of trauma, cardiac and neurosurgical services will play a role as well. |
Tuesday, July 13, 2004
The lead editorial in today's Wall Street Journal goes after Senator Edwards' creative use of tax shelters, including forming something called a "subchapter s" corporation. He paid himself a salary of about $300,000 a year but then took another $25 million over four years as dividends. This kept the Senator from paying about $600,000 in Medicare premiums over that time.
As a political matter, the dodge is especially hypocritical because the income limits on which Medicare taxes are paid were lifted by Democrats in 1993 specifically to hit "the rich," as Mr. Edwards likes to call people in his tax bracket. And the supreme irony? Mr. Edwards has claimed that he set up the subchapter S company to protect himself from legal liability. You know it's time for tort reform when even the trial lawyers say they're afraid of getting sued."Two Americas" indeed. |
Monday, July 12, 2004
What should government-based single-payer healthcare be? Should it be focused on an effort to provide basic coverage for the uninsured, or should it focus on placing everyone in the same boat in the pursuit of "fairness"? Or put more simply, do we want a "one-tier" or a "two-tiered" system. Ronald Bailey at Reason Online asks Why shouldn't more money buy you better health care? It boils down to the "healthcare is different" argument:
In free markets most goods and services are differentiated by quality and customers get what they pay for. The more one pays, the better one expects to be treated. But many bioethicists think that medicine is different-that "health care is a right." But this mentality leads them to the position that we only have a right to the health care the state chooses to give us-and that we ought to be, or at least will be, denied anything better.
Mr Bailey then goes on to give Dr. Caplan credit for agreeing that "access to a minimal package of health care" is society's obligation. A position later claified in the same Fresh Air interview cited by Mr. Bailey:
GROSS: Chris Butler of Independence Blue Cross of southeastern Pennsylvania was saying if you look at some of the national health plans in other countries, you'll see that shadow systems emerge; that people end up wanting to get coverage beyond the basics that you're give in that plan. So you have all these, like, other basically insurance companies that develop, and people with money buy into those companies, so that they could get quicker service or better service than the basic government plan will give you. Sherry Glied, would you agree with that perception?
Ms. GLIED: Well, I think many systems operate very much and very intentionally on that premise. England does, France does. Germany has a private health insurance system. So the question: Is that a failing of those systems, or is it a success of those systems? I think what you might say is, look, we want to have some basic level of care that everyone's entitled to, and those people who want to buy out of that, you know, go ahead and let them do that. We don't even have that basic level of health care for everyone. So I don't think that it's legitimate to complain that other people have two-tier systems when we don't even have a one-tier system for people who are really in need.
GROSS: Art Caplan.
Mr. CAPLAN: Yeah, I think Sherry's on the right track here. It seems to me we have the same buyout, if you will. We have people these days selling boutique medicine. So if you pay an extra fee to your doctor, then your doctor will call you back or make themselves available to you. If you pay that extra amount, you can get little e-mail messages and communications. This whole area of boutique medicine is rapidly growing within our private sector on top of, if you will, the standard benefits. So we've got some of that.
All that said, the greatest enemy I think of getting universal health care insurance is arguments about the ceilings of health care. A lot of people say, 'But if the British have buyouts and the French have buyouts, that isn't fair. Everybody should be in the same boat.' I think the problem is to agree on what's the minimum. Let's agree on what's in coach. Let's agree on what we're going to have in the back of the plane. Then we can argue about first class.
But should you get pretzels and a Coca-Cola in coach or not? Let us examine the changes in TennCare:
In his column Caplan decries recent reform proposals for Tennessee's state Medicaid program. The reforms, known as TennCare, were launched with much ballyhoo 10 years ago. They involve the state government taking its allocation of federal dollars for Medicaid and using it to cover not only those residents who met Medicaid poverty guidelines, but also other poor residents lacking health insurance. As with most any open-ended government entitlement, TennCare is heading for bankruptcy. So the Tennessee legislature passed a reform earlier this year under which medical necessity would be defined as the least costly "adequate care," instead of the traditional standard of "most effective" care.
Although a bit vague, the concept of "adequate care" encompasses such things as requiring doctors to prescribe generic drugs whenever possible; limiting the number of prescriptions to no more than six per month without special permission; requiring co-payments from patients in order to cut down on frivolous visits; and an annual limit on the number of doctor visits. Instead of prescription medicines, TennCare patients will have to buy over-the-counter medications like Prilosec for controlling stomach acid and Allegra for allergies.
In Dr. Caplan's own words:
It is making over its state Medicaid program known as TennCare. If this program gets implemented, many of the poor, elderly, children and disabled in Tennessee who rely on Medicaid will be told simply to get over it....Gov. Phil Bredesen, a former HMO entrepreneur, sees the challenge of health care for the poor in Tennessee in very stark terms. In a speech last February, the governor described the state Medicaid program as nothing more than an open checkbook that is continuously being raided by "doctors and hospitals and advocates" who "decide what is needed."
Well, who should be deciding what is needed for medical treatment if not doctors and hospitals and advocates? Not under TennCare, if the governor gets his way. Bureaucrats, not doctors, will pick how the poor get treated.
Historically, decisions about what drugs or treatments a patient received were chosen by a standard of care known as "medical necessity." Doctors determined what was medically necessary based on local standards of medical practice, and if they did not practice according to this standard they could be found guilty of malpractice. TennCare does away with the established standard and replaces it with a new one - "adequate care." If a bureaucrat in the Tennessee department of health thinks a low-cost drug or treatment, or even no treatment at all, is "adequate," then that is what TennCare will provide.
Under the new definition, preventive care and many pain medications will no longer be funded. And only generic drugs will be available to treat poor kids with life-threatening conditions such as cystic fibrosis, cancer or asthma, and no prescription antihistamines or gastric-acid reducers for anyone of any age. If you want to protest these inadequacies, you might be able to find a doctor willing to plead your case to a special state-established foundation.
Aren't generic drugs just as good? Isn't Big Pharma being greedy and evil for not making more of it's drugs available in generic form? Isn't the main advantage of current prescription antihistamines, their non-sedating nature, more of conveniencece than of effectiveness? You can buy generic Loratadine from CVS for less than fifty cents a pill. 60 Famotidine will cost you $11.99. Which is better, cutbacks like these or reducing the number of people eligible for the program?
BTW, Dr. Caplan is no fan of "concierge medicine" either.
There are several reasons that some oppose a "two-tier" system:
There are concerns that the "secondary market" insurance wil be primarily utilized by younger and healthier individuals. As they become older and sicker they would drop their secondary insurance and fall back into the "single payer" pool. This would increase the burden on the system. The problem I have with that argument is that they would be paying for the single payer system throughout. A good analogy is when parents send their children to private or parochial schools. They pay tuition as well as the school taxes for the public schools in their community.
Support for the system overall will diminish if were are not all in the same boat. Under this theory those that can afford secondary insurance will not feel the need to support the program overall. The same argument is often used in the debate over means-testing for Medicare. This could apply to providers as well. The fear is that the single payer group would be treated as second class citizens and receive second class care.
It's not fair that "the rich" can buy themselves additional coverage that helps them avoid the messy details of a single-payer system. Well the obvious retort is that life isn't fair, but that reeks of sophistry. But then again the "not fair" argument itself is sophomoric and naive. It ignores the way the real world works. Suppose a "one size for all" plan is put into place. How long before offshore insurance companies open up with services provided offshore, much like the "riverboat casinos"? Not very long. What about taxing the premiums or benefits or procedures? While this could satisfy the vindictiveness of some, it would be counter to the fairness argument since it could price many out of the "secondary market"
But he still hasn't come up with a convincing answer to the question: What's ethically wrong with people with means doing "whatever they want" with regard to their health care? They can already do whatever they want with their educations, jobs, housing, food, and so forth. So eager is Caplan to play class warfare by contrasting concierge care with adequate care that he actually misses the main lesson to be learned from TennCare-that any government-run national single payer system would inevitably run up against fiscal limits and impose rationing on everybody. Bureaucrats would then be making health care decisions for us all. But then at least we could share the "solidarity" of all having the same equally inadequate health care.
The question is: are single-payer advocates willing to accept a two or more tier system to insure universal coverage? Because in the United States, that is what it is going to take.
Other views from Galen and Mr. McBride. |
Sunday, July 11, 2004
This Land
Don't be drinking anything when you do, you may damage your monitor. |
Teens not shy about breast implants from The Atlanta Journal-Constitution:
In a Rich's-Macy's changing room, Amanda Ross smiles as she squeezes her sinewy body into an orange nylon tank top.There is an accompanying photograph of the young ladies in question with the following caption:
"I never could have worn this before," she says, sashaying before a full-length mirror.
"You look great," says Paige Bellamy, trying on a white body-hugging shirt.
Ross and Bellamy, friends ages 19 and 18, recently did something once unthinkable for women so young: They got breast implants. They joined a surge of American women, many of them teenagers, lining up for breast enlargement surgery like never before.
Friends Amanda Ross (left), 19, and Paige Bellamy, 18, say their recent breast enlargement surgeries allow them to wear a wider range of clothes. They liken breast implants to a cosmetic change, such as teeth whitening.In a bit of unintended humor the photo has a "click to enlarge" link below it.
These young ladies don't seem to mind the potential consequences. Putting aside the discredited link between silicone implants and connective tissue disorders, these women seem to shrug off any concerns. Concerns including difficulty with mammography, clinical exam and breastfeeding. What about the potential for implants having to be replaced in ten years?
Ross said she hasn't thought a lot about the need to replace the implants, which health officials say is required every 10 years or so. "I'll be an older woman then," she said.But wiser? Only time will tell. There are financial concerns as well. One of the young women financed the $6,000 for her implants over four years with monthly payments of $160. This could impose a significant burden. On the other hand, the chances of repossession are slim, so what have you got to lose? These young ladies are not alone:
Of the 280,401 women who received cosmetic breast implants last year, 11,326  or 4 percent  were under 19, according to the American Society for Aesthetic Plastic Surgery. In previous years, that age group accounted for 1 to 2 percent of the total. Statistics from the American Society of Plastic Surgeons are more conservative, saying 3,841 women under 19 got breast implants last year. That's a higher percentage of its total than in the past two years. Both groups report a doubling of all breast enlargement surgeries over the past five years.The article parades some psychology types who provide the usual boilerplate about society's expectation of women looking like Barbie and the effects that has on "self esteem". Diane Zuckerman, president of the National Center for Policy Research for Women & Families, goes so far as to say, "You don't want them to be making decisions about plastic surgery." I wouldn't go that far but I have serious issues with how these young ladies view a major surgical procedure with comparing to teeth whitening. It's called "informed consent" for a reason but with statements such as this:
Bellamy.... admits that she only pretended to read the waiver forms about possible complications from the surgery. "I didn't want to freak myself out, so I wouldn't read it," she said.I wonder how informed she was. Well you pays your money and you take your chance. A note to Ms. Bellamy's surgeon: clip and save for your malpractice attorney.
My sample suffers from selection bias since my breast patients see me for cancer concerns, but I have yet to have a patient tell me she was happy with her decision to have implants 20-30 years ago. |
Saturday, July 10, 2004
From Friday's Wall Street Journal: After Medtronic Lobbying Push,The FDA Had Change of Heart($$)
In May, an article posted on a prominent medical journal's Web site raised concerns about the long-term safety record of a device to treat bulges in the main artery leading from the heart. Now, all that remains on the site is the title.This has drawn the ire of the study's authors, Dr. Dale Tarvis and Dr. Lazar Greenfield (yes, that Greenfield). Aortic stent-grafting has become big business with Medtronic selling 10,000 AneuRx devices a year to the tune of $200 million. Aortic stent-grafting has fallen victim to the "man with a hammer" trap of medical devices. When the devices were first used their indications were for patients that had severe medical contraindications to conventional aneurysectomy. The importance of this distinction will become clear later. Gradually the indications widened to include those patients with "hostile abdomens" and now to where if a patient has an aneurysm and meets the anatomic guidelines they are offered the procedure. Patients also have begun to demand the procedure.
The article, written by Food and Drug Administration researchers, focused on the AneuRx stent graft, a 5-inch metal-and-fabric cylinder designed to prevent deadly ruptures of the arterial bulges, called aneurysms. It suggested that, over time, a rival treatment -- invasive surgery -- was safer.
The device's maker, Medtronic Inc. of Minneapolis, sharply disputed the study's conclusion, reprising arguments from 2003 when it quietly persuaded the FDA to tone down a public notice on AneuRx's safety. Medtronic objected to the FDA that the authors used confidential data without permission. Its lawyer also threatened the editors of the Journal of Vascular Surgery with "criminal and civil sanctions" if they didn't pull the article from the Web site. In the end, the FDA asked the journal to remove the paper from the site, and in late June the agency officially withdrew it from publication.
The story of Medtronic's vigorous lobbying behind closed doors to modify the conclusions of midlevel FDA specialists offers a rare look at the agency handling a complaint by a big company.
The episode also shines a spotlight on a topic of intense debate: What right does the public have to see the information companies collect about their drugs and medical devices? Today, companies reveal important data to the FDA, but the agency typically releases only the information that it thinks is essential for doctors to know. The idea is to avoid confusion or overreactions and protect companies' proprietary data, which the FDA is generally prohibited from disclosing. Now some critics are calling the system inadequate, citing the example of industry findings about possible dangers of antidepressants to children that weren't published.
In April 2001, two years after AneuRx was approved, the FDA put out a health notice warning of possible problems with the AneuRx and a competing device. One concern was "migration," meaning that the AneuRx might move from the spot where it was implanted. The notice also cited possible leaks in which blood flows outside the device into the aneurysm, thus leaving a continued risk of rupture.That is, the device should only be used in patients with severe medical co-morbidities. The April 2001 warning is here. An excerpt:
The notice didn't answer a crucial question: Do these problems make the AneuRx riskier overall than surgery?
The FDA turned to that question in 2002, preparing a public notice to doctors. Studies and interviews with vascular surgeons indicate that at top hospitals, the short-term mortality rate for surgery is very low. Typically, about 1% to 2% of patients die in such hospitals after the operations. At two highly regarded programs, the University of Michigan and the Cleveland Clinic, the death rate is about 1%. But at community hospitals, the rate is thought to be in the 4% to 6% range.
In an internal 2002 draft of its notice to doctors, FDA officials cited the lower 1% to 2% figure as the most suitable comparison for the AneuRx data being supplied by Medtronic. The hospitals that treated the AneuRx patients included in Medtronic's data were the same type of high-volume hospitals that had good results in surgery, so the authors of the draft considered this an apples-to-apples comparison.
AneuRx fared badly in the face-off. Its aneurysm-related mortality rate in the initial period after implantation surgery -- the period when surgical complications usually arise -- was around 1.5%. That was no better than the open-surgery rate cited in the draft. And the draft said long-term mortality from aneurysm-related causes was worse for the AneuRx than for open surgery.
The FDA draft concluded by recommending that AneuRx not be used when a patient's chance of dying during or shortly after surgery was less than 2%. That would effectively rule out the device for some patients.
AneuRx® System (Medtronic AVE)So Medtronic then brings its guns to bear on the FDA parading experts that attacked the methodology of the trials. The FDA then modified their recommendations and they may be found here. Back to the WSJ
This device has a fabric graft supported along its entire length by a series of metal rings sutured to the graft. The endograft is held in place by the radial force applied by the rings to the patients aorta. FDA is concerned about reports of approximately 25 aneurysm ruptures, as well as other serious adverse events, in patients who have received AneuRx®. Factors thought to be associated with the adverse events, including aneurysm ruptures, include: sub-optimal placement of the graft; endoleak (inadequate proximal seal, collateral vessel retrograde flow, persistent perigraft flow); migration of the main body of the device as well as any attachment cuffs, possibly associated with continuing aortic dilatation; problems with device integrity, due to metal frame fractures, suture breaks, or fabric tears; and aneurysm anatomy. We are working with Medtronic AVE to obtain relevant data that will help us understand how these problems affect the overall risk/benefit assessment of this product.
The tug of war seemed to have been resolved. Then on May 7 of this year, an article called "Aneurysm-related mortality rates in the US AneuRx clinical trial" appeared in the Journal of Vascular Surgery's online preview section. Its authors were Dr. Tavris and two FDA colleagues, as well as Dr. Greenfield, the University of Michigan vascular-surgery professor.....So the lawyers got involved and fired off a letter to the Journal of Vascular Surgery demanding that the article be removed due to "disclosure of ... proprietary information and breach of confidentiality protections are subject to both criminal and civil sanctions which will be pursued vigorously if this situation is not remedied." The article was pulled and there is some finger-pointing between the authors, the editors of the JVS and the FDA about who gave the go-ahead to pull the article.
The paper relied on the same Medtronic data used by the FDA in preparing its public-health notice. The paper revived the 1% to 2% open-surgery death rate, saying a low figure was most relevant for comparison because the AneuRx recipients covered in the data were at institutions that treated lots of aneurysms.
The paper concluded that the mortality rate for patients getting the AneuRx probably exceeded that for surgical patients by three years or more after the treatment, because the AneuRx had little advantage in preventing immediate post-surgical deaths and caused or allowed more problems down the road.
Medtronic responded in a letter to the FDA dated May 20, asserting that the paper's authors were using confidential company data without permission. The letter argued again that the article was underestimating the surgery death rates. Medtronic said it had reached agreement with the FDA about the December public-health notice and the article went "well beyond" the notice, "in some cases reverting to the position[s] in the initial draft ... which we ... believed were wrong and which were ultimately eliminated in the final version" of the notice.
Shenanigans with aortic stent-grafts are not new, as the information about another once-popular system was described in the April 2001 memorandum:
Ancure® system (Guidant)(emphasis mine) That is why when you Google Ancure you get the first two-and-a-half pages are sites for law firms involved in suits. This in addition to a guilty plea and a fine of $92 million.
This device has a flexible, unsupported fabric graft prosthesis that is actively fixed in place on the ends by wire hooks that penetrate the vascular tissue. On 3/16/01, Guidant suspended production and announced a recall of all existing inventory. The company reported to the FDA that they had failed to report many device malfunctions and adverse events, including severe vessel damage associated with problems with the deployment of the device. There were also manufacturing changes that were not properly reported to the FDA. The manufacturer told FDA that an internal audit revealed problems with their complaint handling system, manufacturing quality systems, documentation procedures and training. The FDA is reviewing the firms Corrective Action Plan that addresses these problems. Once we receive evidence that the firm has appropriately changed their systems and procedures, and the FDA has reviewed their regulatory submissions, we will assess whether the product can be returned to the market.
Ironically these companies got to great lengths to insure the competence of the physicians inserting the devices. Before being allowed to place the devices a physician had to attend didactic courses as well as deployment of the endografts in animals and models. When you began to place them in patients you were required to submit your workup (CT and angiograms) to the manufacturer. A representative then was present for your first five insertions. Only then were you allowed to keep grafts "on site". It seems to me that if the company went through so much trouble to keep the device out of the hands of stumblebums, they would be diligent about other aspects of manufacture. Guess not.
The device used by my group now is the Gore Excluder graft. So far they seem to have a good safety record.
If you believe it. |
Friday, July 09, 2004
With my partners going on vacation I was on call Thursday and will be on call four out of the next seven days. Then I am not on call for the rest of the month. It's nice to get it out of the way early. Posting volume will vary inversely to clinical nonsense for the next few days. |

WOULD YOU BUY A USED CAR FROM THIS MEDBLOGGER???
Dr. Ostrovsky of EchoJournal and I have been sharing emails about medblog honesty. He emailed me a few days ago. After some pleasantries about my trauma ultrasound post he writes :
Also, here is something that's been bothering me for sometime. Bloggers have already started to put up medical case reports. I do it. You do it. But here is the problem. How do we know and how do people know that they can trust these case reports and research? One day some crazy medical blogger will put up some lies, and all medbloggers might end up with the spoiled reputation. In my opinion, we have to come up either with some type of code of conduct for medical blogs, or some kind of medical blogger "society". We need to protect our reputation, and we need to look trustworthy even in this early stage of blog evolution. Please let me know what you think. Do you think it is an issue that we even need to be worried about?My reply:
Dr. Ostrovsky:
Your email brings up a good point, who do you trust?
I find it useful to divide the medical reporting in blogs in to two groups: commentary on published material and anecdotal reports.
Commentary on published material, be it in the peer-reviewed literature or in the lay press, is easy to evaluate as any blogger worth their salt will provide, or attempt to provide, a link to the source material. The blogger may editorialize their view about the story and commenters or other bloggers may take them to task. With the source material being linked it becomes an issue of "we report, you decide".
Anecdotal case reports are another case entirely. If I claim that I am cloning people in my garage or performing brain transplants, that doesn't come close to passing the smell test. Other cases are not so clear cut. Claims of "this treatment worked for me so everyone should do it" should be viewed with a jaundiced eye. Stories of patient interactions, such as the one I posted on 6/28, have to be taken in context with other postings. If you think that the blogger is an otherwise straight shooter, then posts such as that may be taken at face value. When discussing clinical scenarios and patients there is, and must be, some "filtering" of the facts to protect confidentiality. What about those of us who post using a pseudonym (including myself)? Can they be trusted?
As far as a "code of ethics" is concerned that would be a nice thing, but probably not practical. Who would write it? Who would enforce it? How? Strongly-worded censure posts? De-linking? My opinion is to let the readers be the guide. If someone puts a great deal of B.S. on their blog people will call them on it, and if it continues they will be ignored.
As with everything on the internet the watchword is: caveat emptor.
Sincerely,
Bard-Parker
Discuss amongst yourselves. |
Thursday, July 08, 2004
A commenter to this post asked:
Are you per chance the writer of a small paper back text from Parkland of the same name? " A Chance to Cut is a Chance to Cure" ???
I had a copy in the 80's while training in Denver, cannot find it now - any around?
I'm afraid not. I have read it (my partner has a copy). The literary and medical genius behind that work is a surgeon named Rip Pfeiffer. They copy my partner has is a third edition from 1983. I could not find it on ebay or at Barnes and Noble.com. Amazon has a copy, overpriced in my opinion. Dr. Pfeiffer has an address for additional copies in the last page of the book. I am unsure if the address is still good. I will write to him at that address and get find if any are available. |
Wednesday, July 07, 2004
N.J. Mulls Cosmetic Surgery Tax
People who have unnecessary cosmetic surgery in the state will soon have to start paying a 6 percent tax for their procedure if Gov. James McGreevey signs a budget that was passed last week by the state Legislature.
It will be the first time a tax has ever been placed on a surgical procedure in America.
The bill, sponsored by Democratic Sen. Wayne R. Bryant and Democratic Assemblyman Joseph Cryan, imposes a 6 percent tax on certain cosmetic medical procedures that are directed at improving the patient's appearance and that do not promote the proper function of the body or prevent or treat illness or disease.
Needless to say, not everyone is happy:
I think it is an extremely unfair taxation on a lot of patients who are not extremely wealthy and often save up for long periods of time for these procedures, said Dr. Frank DiSpaltro, former president of the American Society for Plastic Surgery and a New Jersey physician. Doctors say they are worried that they will lose money and patients because of the new bill. ÂNew Jersey already suffers from having patients go to New York or Pennsylvania for healthcare  and I think this is going to further fuel that exodus, said Dr. Martin Moskovitz, a plastic surgeon in West Orange
This is a tax that is relatively painless for the politicians. The majority of those who have these procedures pay out-of-pocket and they will pay without much complaint, or competitive pressures will force the plastic surgeons to eat the cost. The revenues? Well there's the rub:
The tax is expected to increase state revenues by $26 million. The money is intended to go to charitable or uninsured patients, although that is not specified in the bill.That's my problem with this. If the money was going to coverage, that's one thing, but if it's not then the fairness is called into question. |






